卓越班の成果
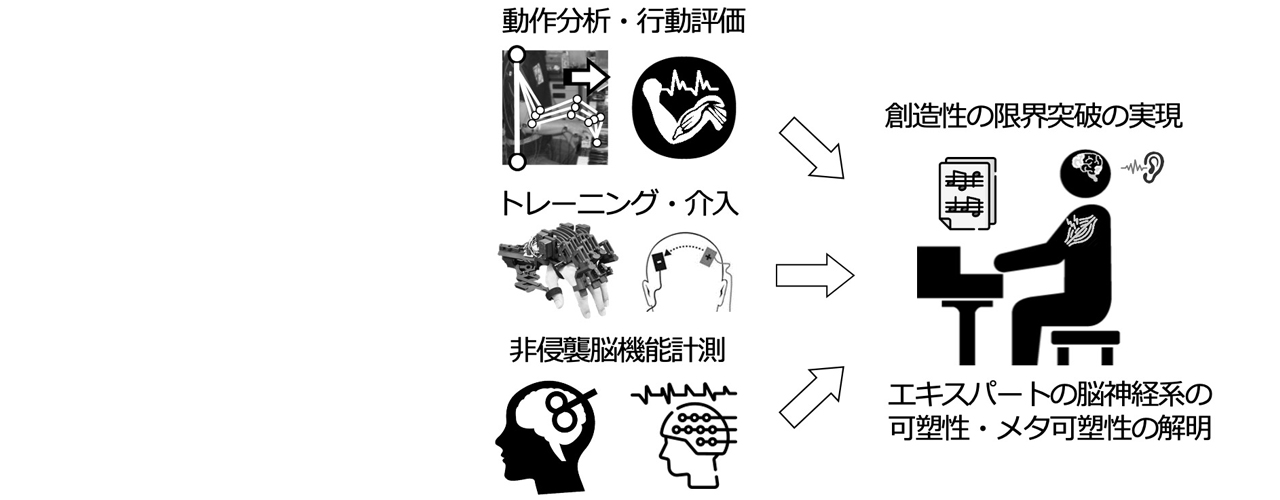
卓越班の代表である古屋は,エキスパートの技能の獲得・洗練・喪失・再獲得に関わる脳と身体のメカニズムの解明と,最適なトレーニング・リハビリテーションの創出を目指し,音楽家を対象とした研究に取り組んできた.特に,音楽家のスキルの評価や(①②),背後にある神経機序の解明(③),スキルの天井効果の証明(④),スキルの個人差を生み出す要因の同定(⑤)や,アガリや局所性ジストニアによるスキルの失調と背景機序の解明(⑥,⑦,⑧),スキルの限界を突破するトレーニングやリハビリテーションの開発と評価(⑨,⑩)を行ってきた.その結果,ドイツ研究振興会(DFG)よりHeisenberg Fellowshipや,文部科学省卓越研究員などを受賞してきた.本領域では、動作分析や行動・神経介入手法,非侵襲脳機能計測とデータサイエンス技術を利用し,エキスパートのスキルの限界を規定する神経メカニズムの解明とその突破を目指す.
基礎となる主なこれまでの研究
- ①Furuya S, Kinoshita H. Neuroscience, 2008
- ②Furuya S et al. J Neurophysiol. 2011
- ③Hirano et al. Cerebral Cortex, 2020
- ④Furuya S et al. The Journal of Neuroscience, 2014
- ⑤Furuya S. Current Opinions in Behavioral Sciences, 2018
- ⑥Furuya S et al. The Journal of Physiology, 2018
- ⑦Kotani and Furuya. J Neurophysiol. 2018
- ⑧Furuya S et al. Movement Disorders, 2020
- ⑨Furuya S and Yokota K. J Neurophysiol, 2018
- ⑩Furuya S et al. Annals of Neurology, 2014
本領域で得た主な成果
- Hirano M et al. Science Advances, 2020
- Kimoto Y et al. Cerebral Cortex, 2021
- Furuya S et al. Communications Biology, 2021
- Oku T and Furuya S. Sensors, 2022
- Hirano M and Furuya S. Scientific Reports, 2022
- Furuya S et al. Annals of NY Academy of Sciences, 2023
- Muramatsu K et al. Scientific Reports, 2022
健常班の成果
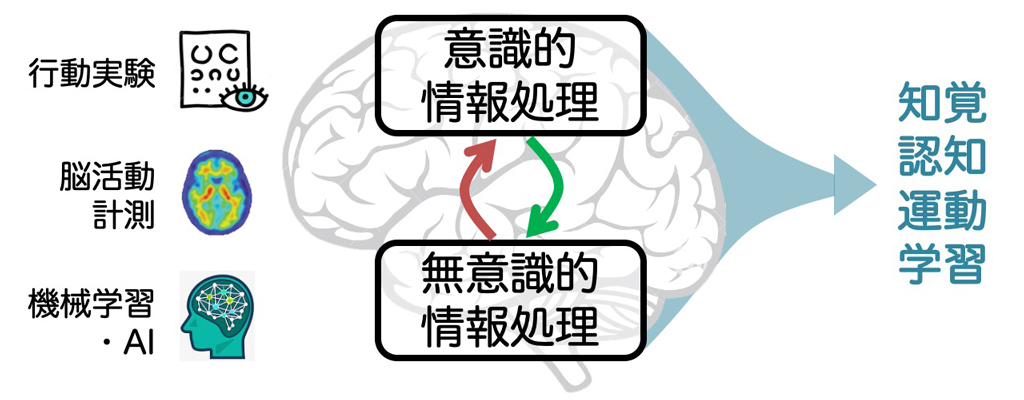
健常班の代表である柴田は、ヒトの能力や学習、認知を支える脳の無意識的情報処理メカニズムに焦点を当て研究を行ってきた(①②③)。特に、行動実験、脳活動計測、機械学習を組み合わせたユニークなアプローチにより、脳の無意識的情報処理に働きかけることで知覚(④)、認知(⑤)、運動能力(⑥)、学習(⑦)の促進が可能であることを示してきた。またリアルタイム脳活動測定と人工知能技術を組み合わせて開発したDecoded Neurofeedback (DecNef) を用いることで、脳の賦活パターンと知覚・認知機能の関係を明らかにしてきた(⑧⑨⑩)。DecNefは様々な大型研究プロジェクトの中核技術として用いられ、柴田は2021年度の日本学術振興会賞を受賞している。本領域では、行動実験、脳刺激、DecNefを有機的に組み合わせ、ヒトの能力限界を決める脳メカニズムの解明とその突破を目指す。
基礎となる主なこれまでの研究
- ①Shibata K et al., Nature Neuroscience, 2017.
- ②Bang JW et a., Nature Human Behavior, 2018.
- ③Izuma K et al., Journal of Personality and Social Psychology, 2018.
- ④Shibata K et al., Current Biology, 2012.
- ⑤Choi H et al., Proceedings of the National Academy of Sciences of USA, 2012.
- ⑥Amano et al., Current Biology, 2016.
- ⑦Shibata K et al., Vision Research, 2009.
- ⑧Shibata K et al., Science, 2011.
- ⑨Shibata K et a., PLoS Biology, 2016.
- ⑩Koizumi A et al., Nature Human Behavior, 2016.
本領域で得た主な成果
- Marzoll A et al. iScience, 2022
- Takado Y et al., Journal of Cerebral Blood Flow & Metabolism, 2022
- Zhiyan Wang Z et al., Journal of Vision, 2021
- Cortese A et al, Scientific Data, 2021.
- Shibata K, A book chapter in Book “fMRI Neurofeedback”, 2021.
- Kasahara S et al., Proceedings of the 2021 CHI Conference on Human Factors in Computing Systems.
倫理班の成果
倫理班の代表である中澤栄輔は、これまで哲学・倫理学の方法論(特に科学哲学・現象学・心の哲学)を用いて生命・医療倫理学領域で研究活動を行っており、医療・医科学技術と人間・社会との関係を主題にしてきた。特に脳神経倫理の領域においてニューロフィードバックの安全性と倫理性を論じ、当該領域の議論をリードした(①)。また我が国の脳神経倫理の課題の取りまとめを行い(②③④)、当該領域の発展に寄与してきた。
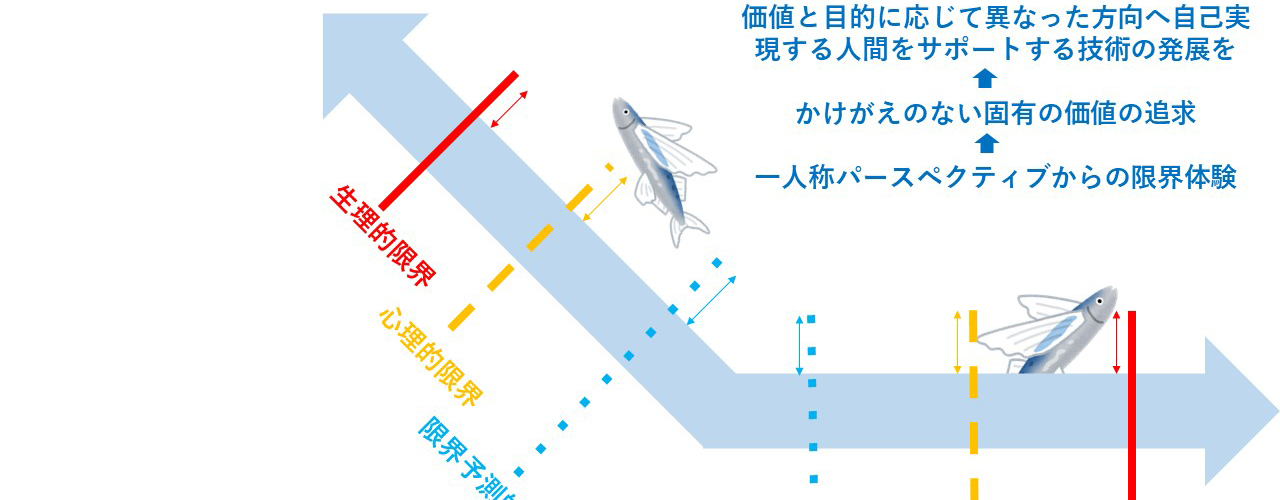
本領域では、限界突破に関する概念整理を行った後、限界突破技術の社会応用を見越して潜在的な社会的・人間的問題を洗い出すため、質問紙調査を行った。1つ目の結果は、限界概念の明確化に関連する。限界体験と、限界を認識することによる抑制体験の有無とその具体的内容について一般市民の現状を把握したところ、限界体験を有する者がおよそ30%、限界抑制体験を有するものがおよそ30%だった。研究参加者は一人称パースペクティブからの限界体験の報告をしており、限界体験と抑制体験およびその突破に関する主観的側面からのアプローチの可能性が示唆された。また、限界体験および抑制体験に関連する因子を抽出し、限界突破技術の社会的ニーズの把握の一助とした(⑤)。2つ目の結果は限界突破技術を用いることについてのユーザーの希望とそれに影響する他者の行為の関係について示唆を得た。日本では同調圧力が大きな影響を持ち、限界突破技術が実現したとしても利用が自重される傾向が明らかになった。また限界突破技術の円滑な社会受容に際し、開発の段階から将来のユーザーの意見を取り入れていくこと(パブリックインボルブメント)の重要性が示された(⑥)
本領域では、限界突破に関する概念整理を行った後、限界突破技術の社会応用を見越して潜在的な社会的・人間的問題を洗い出すため、質問紙調査を行った。1つ目の結果は、限界概念の明確化に関連する。限界体験と、限界を認識することによる抑制体験の有無とその具体的内容について一般市民の現状を把握したところ、限界体験を有する者がおよそ30%、限界抑制体験を有するものがおよそ30%だった。研究参加者は一人称パースペクティブからの限界体験の報告をしており、限界体験と抑制体験およびその突破に関する主観的側面からのアプローチの可能性が示唆された。また、限界体験および抑制体験に関連する因子を抽出し、限界突破技術の社会的ニーズの把握の一助とした(⑤)。2つ目の結果は限界突破技術を用いることについてのユーザーの希望とそれに影響する他者の行為の関係について示唆を得た。日本では同調圧力が大きな影響を持ち、限界突破技術が実現したとしても利用が自重される傾向が明らかになった。また限界突破技術の円滑な社会受容に際し、開発の段階から将来のユーザーの意見を取り入れていくこと(パブリックインボルブメント)の重要性が示された(⑥)
基礎となる主なこれまでの研究
- ① Nakazawa E et al. AJOB Neurosicience, 2016
- ② Rommelfanger et al. Neuron 2018
- ③ Sadato N et al. Neuron, 2019
本領域で得た主な成果
- ④ Nakazawa E et al. Neuroscience Research, 2022
- ⑤ Nakazawa E et al. J, 2022
- ⑥ Nakazawa E et al. BioTech, 2022
病態班の成果
基礎となる主なこれまでの研究
- Time-dependent central compensatory mechanisms of finger dexterity after spinal cord injury. Nishimura Y, Onoe H, Morichika Y, Perfiliev S, Tsukada H, Isa T. Science. 2007 Nov 16;318(5853):1150-5. IF:47.728
- Asubcortical oscillatory network contributes to recovery of hand dexterity after spinal cord injury. Nishimura Y, Morichika Y,Isa T. Brain. 2009 Mar; 132(Pt 3):709-21. doi:10.1093/brain/awn338. IF:13.501
- The Ventral Striatum is a Key Node for Functional Recovery of Finger Dexterity After Spinal Cord Injury in Monkeys. Suzuki M, Onoe K, Sawada M, Takahashi N, Higo N, Murata Y, Tsukada H, Isa T, Onoe H, Nishimura Y. Cereb Cortex. 2020 May 14;30(5):3259- 3270. pii:bhz307. doi: 10.1093/cercor/bhz307. IF:5.357
- Dynamic Reorganization of Motor Networks During Recovery from Partial Spinal Cord Injury in Monkeys. Chao Z. C., Sawada M, Isa T, Nishimura Y. Cereb Cortex. 2019 Jul 5;29(7):3059-3073. doi: 10.1093/cercor/bhy172. IF:5.357
- Function of nucleus accumbens in motor control during recovery after spinal cord injury. Sawada M, Kato K, Kunieda T, Mikuni N, Miyamoto S, Onoe H, Isa T, Nishimura Y. Science, 2 October 2015:Vol. 350 no. 6256 pp. 98-101, DOI: 10.1126/science.aab3825. IF:47.728
- Bypassing stroke-damaged neural pathways via a neural interface induces targeted cortical adaptation. Kato K, Sawada M, Nishimura Y. Nat Commun. 2019 10:4699 https://doi.org/10.1038/s41467-019-12647-y. IF:14.919
- Spike-timing dependent plasticity in primate corticospinal connections induced during free behavior. Nishimura Y, Perlmutter SI, Ryan WE, Fetz EE. Neuron. 2013 Dec 4; 80(5):1301-9. doi:10.1016/j.neuron.2013.08.028. IF:18.688
- Restoration of upper limb movement via artificial corticospinal and musculospinal connections in a monkey with spinal cord injury. Nishimura Y, Perlmutter SI, *Fetz EE. Front Neural Circuits. 2013 Apr 11; 7:57. doi: 10.3389/fncir.2013.00057. IF:3.342
- Compensatory changes at the cerebral cortical level after spinal cord injury. Nishimura Y, Isa T. Neuroscientist. 2009 Oct;15(5):436-44. doi: 10.1177/1073858408331375. IF:7.52
本領域で得た主な成果
- Suzuki M, Onoe K, Sawada M, Takahashi N, Higo N, Murata Y, Tsukada H, Isa T, Onoe H, Nishimura Y. Cereb Cortex. 2020. 30(5):3259- 3270 doi: 10.1093/cercor/bhz307
- Suzuki M, Inoue KI, Nakagawa H, Ishida H, Kobayashi K, Isa T, Takada M, Nishimura Y. J Physiol. 2022, 600(7):1731-1752. doi: 10.1113/JP282429
- Suzuki M, Nishimura Y. Front Sys Neurosci., 2022, 29, https://doi.org/10.3389/fnsys.2022.979272
- Usuda N, Sugawara S.K, Fukuyama H,Nakazawa K, Amemiya K, Nishimura Y. Neuroscience Research, 2022, doi:10.1016/j.neures.2022.06.008
- Insausti-Delgado A, Lopez-Larraz E, Nishimura Y, Ziemann U, Ramos-Murguialday A. Frontiers in Bioengineering and Biotechnology, 2022. doi: 10.3389/fbioe.2022.975037
- Kaneshige M, Obara K, Suzuki M, Tazoe T, Nishimura Y. eLife, 2022. 10.7554/eLife.78346
- Umeda T, Isa T, Nishimura Y. PNAS, 2022, doi: 10.1073/pnas.2208353119
全成果リスト(当該領域の期間中)
本領域で得た成果に紐づく全論文
-
Musician’s Dystonia: Family history as a predictor for onset and course of the disease. Johanna Doll, Andre Lee, Shinichi Furuya, Bernhard Haslinger, Eckart Altenmuller. Movement Disorders, 2023. (in press) doi: 10.1002/mds.29448
-
Reliability and Validity of the Embouchure Dystonia Severity Rating Scale. Thomas Mantel, Andre Lee, Eckart Altenmuller, Shinichi Furuya, Masanori Morise, Bernhard Haslinger. Journal of Movement Disorders, 2023. 16(2):191-195
-
Passive somatosensory training enhances motor skill of piano playing in adolescent and adult pianists. Shinichi Furuya, Ryuya Tanibuchi, Hayato Nishioka, Yudai Kimoto, Masato Hirano, Takanori Oku. Annals of the New York Academy of Science, 2023. 1519(1): 167-172
-
中枢神経損傷に対する人工神経接続を用いたニューロリハビリテーション. 田添歳樹, 河合一武, 西村幸男. 脳神経内科, 2023. 第98巻第5号:677-683. 2023
-
意欲が身体運動に影響を及ぼす神経基盤. 鈴木迪諒, 西村幸男. 体育の科学. 2023. 第73巻5月号, 296-300, May 1,
-
Plyometric training enhances strength and precision of the finger movements in pianists. Kaito Muramatsu, Takanori Oku, Shinichi Furuya. Scientific Reports, 2022. 12: 22267
-
Corticospinal interface to restore voluntary control of joint torque in a paralyzed forearm following spinal cord injury in non-human primates. Kei Obara, Miki Kaneshige, Michiaki Suzuki, Osamu Yokoyama, Toshiki Tazoe, Yukio Nishimura. Front. Neurosci, 2023. 17:1127095. doi: 10.3389/fnins.2023.1127095.
-
The phase of plasticity-induced neurochemical changes of high-frequency repetitive transcranial magnetic stimulation are different from visual perceptual learning. Shang-Hua N. Lin, Yun R. Lien, Kazuhisa Shibata, Yuka Sasaki, Takeo Watanabe, Ching-Po Lin, Li-Hung Chang. Scientific Reports. 2023. 5720. doi: 10.1038/s41598-023-32985-8
-
第10章ニューロフィードバックは精神疾患の治療に応用できるか (「心の病」の脳科学 なぜ生じるのか、どうすれば治るのか),柴田和久(分担執筆),林(高木)朗子,加藤忠史(著).2023.
-
Non-invasive brain-spine interface: Continuous control of trans-spinal magnetic stimulation using EEG. Insausti-Delgado A, López-Larraz E, Nishimura Y, Ziemann U and Ramos-Murguialday A. Front. Bioeng. Biotechnol, 2022. 10:975037. doi: 10.3389/fbioe.2022.975037
-
Quantitative comparison of corticospinal tracts arising from different cortical areas in humans. Usuda N, Sugawara S, Fukuyama H, Nakazawa K, Amemiya K, Nishimura Y. Neuroscience Research, 2022. 183: 30-49. doi: 10.1016/j.neures.2022.06.008
-
Tuning of motor outputs produced by spinal stimulation during voluntary control of torque directions in monkeys. Kaneshige M, Obara K, Suzuki M, Tazoe T, Nishimura Y. eLife, 2022, doi: 10.7554/eLife.78346
-
Origin of Multisynaptic Corticospinal Pathway to Forelimb Segments in Macaques and Its Reorganization After Spinal Cord Injury. Taihei Ninomiya, Hiroshi Nakagawa, Ken-ichi Inoue, Yukio Nishimura, Takao Oishi, Toshihide Yamashita, Masahiko Takada. Frontiers in Neural Circuit, 2022. 16:847100. doi: 10.3389/fncir.2022.847100
-
Decrease in signal-related activity by visual training and repetitive visual stimulation, (*co-first author) Marzoll A*, Shibata K*, Toyoizumi T, Chavva I, & Watanabe T. iScience, 2022, 25(12):105492.
-
The dorsal premotor cortex encodes the step-by-step planning processes for goal-directed motor behavior in humans. Yoshihisa Nakayama, Sho K Sugawara, Masaki Fukunaga, Yuki H Hamano, Norihiro Sadato, Yukio Nishimura. Neuroimage, 2022, doi: 10.1016/j.neuroimage.2022.119221
-
A multisynaptic pathway from the ventral midbrain toward spinal motoneurons in monkeys. Michiaki Suzuki, Ken-ichi Inoue, Hiroshi Nakagawa, Hiroaki Ishida, Kenta Kobayashi, Tadashi Isa, Masahiko Takada, and Yukio Nishimura. The Journal of Physiology (London), 2022, 600(7):1731-1752
-
MRS-measured glutamate versus GABA reflects excitatory versus inhibitory neural activities in awake mice. Takado Y*, Takuwa H*, Sampei K, Urushihata T, Takahashi M, Shimojo M, Uchida S, Nitta N, Shibata S, Nagashima K, Ochi Y, Ono M, Maeda J, Tomita Y, Sahara N, Near J, Aoki I, Shibata K, & Higuchi M. Journal of Cerebral Blood Flow & Metabolism, 2022, 42(1):197-212
-
Impaired feedforward control of movements in pianists with focal dystonia. Ken Takiyama, Shuta Mugikura, Shinichi Furuya. Frontiers in Neurology 13:983448. 2022. doi: 10.3389/fneur.2022.983448
-
Multisensory interactions on auditory and somatosensory information in expert pianists. Masato Hirano, Shinichi Furuya. Scientific Reports, 2022 12, 12503. doi: 10.1038/s41598-022-16618-0
-
Sensorimotor incoordination in musicians’ dystonia. Shinichi Furuya and Takanori Oku. A book chapter in Basic and Translational Applications of the Network Theory for Dystonia (edited by Aasef Shaikh and Anna Sadnicka). Springer, 2023 (in press).
-
Adaptation of the corticomuscular and biomechanical systems of pianists. Yudai Kimoto, Masato Hirano, Shinichi Furuya. Cerebral Cortex 2022. 32(4): 709-724.
-
Individual experiences with being pushed to limits and variables that influence the strength to which these are felt: A cross-sectional survey study. Nakazawa E, Mori K, Akabayashi A. J 2022. ; 5(3):358-368.
-
The neuroethics of memory’s social value: To what extent can neurotechnologies that manipulate memory be permitted? Nakazawa E, Tachibana K, Yamamoto K, Akabayashi A. Journal of Cognition and Neuroethics, 2022. 9 (1): 1–11.
-
The way forward for neuroethics in Japan: A review of five topics surrounding present challenges. Nakazawa E, Fukushi T, Tachibana K, Uehara R, Arie F, Akter N, Maruyama M, Morita K, Araki T, Sadato N. Neuroscience Research in print. 2022. doi: 10.1016/j.neures.2022.07.006
-
Temporal dynamics of the sensorimotor convergence underlying voluntary limb movement. Umeda T, Isa T, Nishimura Y. Proceedings of the National Academy of Sciences, 2022, 119(48):e2208353119.
-
Activation of human spinal locomotor circuitry using transvertebral magnetic stimulation. Kawai K, Tazoe T, Yanai T, Kanosue K, Nishimura Y. Frontiers in Human Neuroscience. 2022. 16:1016064.
-
人工神経接続を用いた脳・脊髄損傷後の身体運動機能の再建. 田添歳樹, 西村幸男. Brain and Nerve. 2022. Vol.74 No.9, 1111-1116,
-
The ventral striatum contributes to the activity of the motor cortex and motor outputs in monkeys. Suzuki M, Nishimura Y. Frontiers in Systems Neuroscience, 16:979272.
-
A multisynaptic pathway from the ventral midbrain to spinal motoneurons in monkeys. Suzuki M, Inoue K, Nakagawa H, Ishida H, Kobayashi K, Isa T, Takada M, Nishimura Y. The Journal of Physiology, 2022. 600(7):1731-1752.
-
A cross-sectional study of attitudes toward willingness to use enhancement technologies: implications for technology regulation and ethics. Nakazawa E, Mori K, Udagawa M, Akabayashi A. BioTech, 2022. 11(3), 21. doi:10.3390/biotech11030021.
-
Noncontact and High-Precision Sensing System for Piano Keys Identified Fingerprints of Virtuosity. Takanori Oku, Shinichi Furuya. Sensors, 2022, 22(13), 4891. doi: 10.3390/s22134891
-
中澤栄輔.脳神経倫理の展開と情動を操作する技術―MRIニューロフィードバックに着目して.わが国における神経法学の基盤研究―法学・医学・心理学の協働―〈神経科学・心理学篇〉27–32. 2022年
-
Visual perceptual learning of a primitive feature in human V1/V2 as a result of unconscious processing, revealed by decoded functional MRI neurofeedback (DecNef). Wang Z, Tamaki M, Frank SM, Shibata K, Worden MS, Yamada T, Kawato M, Sasaki Y, & Watanabe T, Journal of Vision, 2021, 21(8):24.
-
The DecNef collection, fMRI data from closed-loop decoded neurofeedback experiments, Cortese A, Tanaka SC, Amano K, Koizumi A, Lau H, Sasaki Y, Shibata K, Taschereau-Dumouchel V, Watanabe T, & Kawato M. Scientific Data. 2021, 65.
-
Mechanisms of fMRI neurofeedback, Shibata K, A book chapter in fMRI Neurofeedback (edited by Michelle Hampson). Academic Press, 2021.
-
Preserving Agency During Electrical Muscle Stimulation Training Speeds up Reaction Time Directly After Removing EMS, Kasahara S, Takada K, Nishida J, Shibata K, Shimojo S, & Lopes P, Proceedings of the 2021 CHI Conference on Human Factors in Computing Systems, 1-9.
-
Neurotechnology for Bypassing Damaged Neural Pathways. Kato K, Nishimura Y. Journal of Aging Science, 2021 DOI: 10.35248/2329-8847.21.9.246
-
Back to feedback: aberrant sensorimotor control in music performance under pressure. Shinichi Furuya*, Reiko Ishimaru*, Takanori Oku, Noriko Nagata. Communications Biology 2021. 4(1): 1367
-
Nucleus accumbens as the motivation center is essential for functional recovery after spinal cord injury. Michiaki Suzuki, Yukio Nishimura. Journal of Rehabilitation Neurosciences, 2021, 21: 23-28
-
The Ventral Striatum is a Key Node for Functional Recovery of Finger Dexterity After Spinal Cord Injury in Monkeys. Suzuki M, Onoe K, Sawada M, Takahashi N, Higo N, Murata Y, Tsukada H, Isa T, Onoe H, Nishimura Y. Cerebral Cortex. 2020, 30(5):3259-3270. doi: 10.1093/cercor/bhz307.
-
Role of the nucleus accumbens in functional recovery from spinal cord injury. Sawada M, Nishimura Y. Neuroscience Research 2021 Nov;172:1-6. doi: 10.1016/j.neures.2021.04.006.
-
Assessment of safety of self-controlled repetitive trans-vertebral magnetic stimulation. Sasada S, Kadowaki S, Tazoe T, Murayama T, Kato K, Nakao Y, Matsumoto H, Nishimura Y, Ugawa Y. Clinical Neurophysiology 2021. 132(12):3166-3176. doi: 10.1016/j.clinph.2021.09.016.
-
Changes in beta and high-gamma power in resting-state electrocorticogram induced by repetitive transcranial magnetic stimulation of primary motor cortex in unanesthetized macaque monkeys. Honda Y, Nakamura S, Ogawa K, Yoshino R, Tobler PN, Nishimura Y, Tsutsui KI. Neuroscience Research. 2021. 171:41-48. doi: 10.1016/j.neures.2021.02.002.
-
Cerebellar outputs contribute to spontaneous and movement-related activity in the motor cortex of monkeys. Sano N, Nakayama Y, Ishida H, Chiken S, Hoshi E, Nambu A, Nishimura Y. Neuroscience Reearch. 2021. 164:10-21. doi: 10.1016/j.neures.2020.03.010.
-
閉回路型脊髄刺激によるニューロモジュレーションの誘導. 田添歳樹, 西村幸男. 運動器リハビリテーション, 2021, 32(3), 255-262
-
中澤栄輔.ニューロモデュレーションの医療倫理.精神医学 63巻12号:1767-1774. 2021年
-
Artificial cortico-muscular connection via neural interface to regain volitional control of limb movements. Kato K, Nishimura Y. Journal of Rehabilitation Neurosciences. 2020, 20(1), 1–6. doi.org/10.24799/jrehabilneurosci.200731
-
The Ventral Striatum is a Key Node for Functional Recovery of Finger Dexterity After Spinal Cord Injury in Monkeys. Suzuki M, Onoe K, Sawada M, Takahashi N, Higo N, Murata Y, Tsukada H, Isa T, Onoe H, Nishimura Y. Cerebral Cortex. 2020, 14;30(5):3259-3270. doi: 10.1093/cercor/bhz307.
-
Stimulus outputs induced by subdural electrodes on the cervical spinal cord in monkeys. Kato K, Nishihara Y, Nishimura Y. Journal of Neural Engineering. 2020;17(1):016044. doi: 10.1088/1741-2552/ab63a3.
-
Overcoming the ceiling effects of experts’ motor expertise through active haptic training. Hirano M, Sakurada M, Furuya S. Science Advances. 2020, 6: eabd2558
-
Soft exoskeleton glove with human anatomical architecture: production of dexterous finger movements and skillful piano performance. Takahashi N, Furuya S, Koike H. IEEE Transaction on Haptics. 2020. 13(4) 679-690
-
Skillful and pathological movement coordination in musical performance. Furuya S, Oku T, Kimoto Y, Nishioka H, Hirano M. Advances in Exercise & Sports Physiology. 2020. 26(2) 23-26
-
Aberrant somatosensory-motor adaptation in musicians’ dystonia. Furuya S*, Lee A*, Oku T, Altenmüller E. Movement Disorders. 2020. 35(5) 808-815
-
Specialized somatosensory-motor integration functions in musicians. Hirano M, Kimoto Y, Furuya S. Cerebral Cortex. 2020. 30(3) 1148-1158
-
Non-invasive Neuromodulation-基礎・検査・治療 B.検査と治療 歩行障害. 田添歳樹, 兼重美希, 西村幸男. Clinical Neuroscience. 38巻1号: 80-84. 2020年
-
人工神経接続の臨床応用 田添歳樹, 加藤健治, 西村幸男 医学のあゆみ(医歯薬出版株式会社)275巻12・13号, 1271-1274, 2020
-
意欲は身体運動に影響を与えるのか. 菅原 翔, 鈴木迪諒, 西村幸男. Clinical Neuroscience別冊. Vol. 36 No.6, 740-742, 2020
-
中澤栄輔.臨床研究の歴史と研究倫理.整形外科 71巻6号:598–601. 2020年
本領域の基礎となる過去の全論文
[卓越]
- Kahori Kita, Shinichi Furuya, Rieko Osu, Takashi Sakamoto, Takashi Hanakawa (2021) Aberrant Cerebello-Cortical Connectivity in Pianists With Focal Task-Specific Dystonia. Cerebral Cortex 31(10): 4853–4863
- Shinichi Furuya, Reiko Ishimaru, Noriko Nagata (2021) Factors of choking under pressure in musicians. PLoS One 16(1) e0244082
- Masato Hirano, Mizuha Sakurada, Shinichi Furuya (2020) Overcoming the ceiling effects of experts’ motor expertise through active haptic training. Science Advances 6(47) eabd2558
- Nobuhiro Takahashi, Shinichi Furuya, Hideki Koike (2020) Soft exoskeleton glove with human anatomical architecture: production of dexterous finger movements and skillful piano performance. IEEE Transaction on Haptics 13(4): 679-690
- Shinichi Furuya*, André Lee*, Takanori Oku, Eckart Altenmüller (2020) Aberrant somatosensory-motor adaptation in musicians’ dystonia. Movement Disorders 35(5) 808-815
- Masato Hirano, Yudai Kimoto, Shinichi Furuya (2020) Specialized somatosensory-motor integration functions in musicians. Cerebral Cortex 30(3) 1148-1158
- Yudai Kimoto, Takanori Oku, Shinichi Furuya (2019) Neuromuscular and biomechanical functions subserving finger dexterity in musicians. Scientific Reports 9:12224
- Takanori Oku, Shinichi Furuya (2019) Neuromuscular incoordination in musicians’ dystonia. Parkinsonism & Related Disorders 65: 97-104
- Kazumasa Uehara, Shinichi Furuya, Hidemi Numasawa, Kahori Kita, Takashi Sakamoto, Takashi Hanakawa (2018) Distinct roles of brain activity and somatotopic representation in pathophysiology of focal dystonia. Human Brain Mapping 40(6) 1738-1749 Kahori Kita*, Jaroslav Rokicki*, Shinichi Furuya, Takashi Sakamoto, Takashi Hanakawa (2018) Resting-state basal ganglia connectivity codes a motor musical skill and its disruption due to a disease process. Movement Disorders 33(9) 1472-1480
- Shinichi Furuya*, Sayuri Yokota* (2018) Temporal exploration in sequential movements shapes efficient neuromuscular control. Journal of Neurophysiology 120(1) 196-210
- Shinichi Furuya*, Kazumasa Uehara*, Takashi Sakamoto, Takashi Hanakawa (2018) Aberrant cortical excitability explains the loss of hand dexterity in musician’s dystonia. The Journal of Physiology 596(12) 2397-2411
- Shuntaro Kotani, Shinichi Furuya (2018) State anxiety disorganizes finger movements during musical performance. Journal of Neurophysiology 120(2) 439-451
- Shinichi Furuya*, Yuta Furukawa*, Kazumasa Uehara, Takanori Oku (2018) Probing sensory-motor integration during musical performance. Annals of the New York Academy of Sciences 1423(1) 211-218
- Masato Hirano, Shinji Kubota, Shinichi Furuya, Yoshiki Koizume, Shinya Tanaka, and Kozo Funase (2018) The acquisition of skilled finger movements is accompanied by the reorganization of the corticospinal system. Journal of Neurophysiology 119(2):573-584
- Shinichi Furuya (2018) Individual differences in sensorimotor skills among musicians. Current Opinion in Behavioral Sciences 20: 61-66
- Yuta Furukawa, Kazumasa Uehara, Shinichi Furuya (2017) Expertise-dependent motor somatotopy of music perception. Neuroscience Letters 650: 97-102
- Sarah Pirio Richardson, Eckart Altenmüller, Katharine Alter, Ron L. Alterman, Robert Chen, Steven Frucht, Shinichi Furuya, Joseph Jankovic, H. A. Jinnah, Teresa J. Kimberley, Codrin Lungu, Joel S. Perlmutter, Cecília N. Prudente, Mark Hallett (2017) Research Priorities in Limb and Task-specific Dystonias. Frontiers in Neurology 8: 170
- Takanori Oku*, Shinichi Furuya* (2017) Skillful force control in expert pianists. Experimental Brain Research 235(5): 1603-1615
- Eckart Altenmüller, Shinichi Furuya (2016) Brain plasticity and the concept of metaplasticity in skilled musicians. Advances in Experimental Medicine and Biology 957:197-208
- Moe Hosoda, Shinichi Furuya (2016) Shared somatosensory and motor functions in musicians. Scientific Reports 6: 37632
- Kenta Tominaga, Andre Lee, Eckart Altenmüller, Fumio Miyazaki, Shinichi Furuya (2016) Kinematic origins of motor fluctuation in expert pianists. PLoS One 11(8):e0161324
- Christos Ioannou, Shinichi Furuya, Eckart Altenmüller (2016) The impact of stress on motor performance in skilled musicians suffering from focal dystonia: Physiological and psychological characteristics. Neuropsychologia 85:226-236
- Andre Lee, Jacob Voget, Shinichi Furuya, Masanori Morise, Eckart Altenmüller (2016) Quantification of sound instability in embouchure tremor based on the time varying fundamental frequency. Journal of Neural Transmission 123(5):515-521
- Shinichi Furuya, Takashi Hanakawa (2016) The curse of motor expertise: use-dependent focal dystonia as manifestation of maladaptive changes in body representation. Neuroscience Research 104: 112-119
- Shinichi Furuya, Takanori Oku, Fumio Miyazaki, Hiroshi Kinoshita (2015) Secrets of virtuoso: neuromuscular attributes of motor virtuosity in expert musicians. Scientific Reports 5:15750
- Shinichi Furuya, Kenta Tominaga, Fumio Miyazaki, Eckart Altenmuller (2015) Losing the dexterity: patterns of impaired coordination of finger movements in musician’s dystonia. Scientific Reports 5:13360
- Shinichi Furuya, Eckart Altenmüller (2015) Acquisition and reacquisition of motor coordination in musicians. Annals of the New York Academy of Sciences 1337: 118–124
- Sara Winges, Shinichi Furuya (2015) Distinct digit kinematics by professional and amateur pianists. Neuroscience 284: 643–652
- Shinichi Furuya, Matthias Klaus, Michale Nitsche, Walter Paulus, Eckart Altenmüller (2014) Ceiling effects prevent further improvement of transcranial stimulation in skilled musicians. The Journal of Neuroscience 34(41):13834 – 13839
- Floris van Vugt, Shinichi Furuya, Henning Vauth, Hans-Christian Jabusch, Eckart Altenmüller (2014) Playing beautifully when you have to be fast: spatial and temporal symmetries of movement patterns in skilled piano performance at different tempi. Experimental Brain Research 232(11): 3555-3567
- André Lee*, Shinichi Furuya*, Masanori Morise, Peter Iltius, Eckart Altenmüller (2014) Quantification of instability of tone production in embouchure dystonia. Parkinsonism & RelatedDisorders 20(11):1161-1164
- Shinichi Furuya, Ayumi Nakamura, Noriko Nagata (2014) Acquisition of individuated finger movements through musical practice. Neuroscience 275C: 444-454
- Shinichi Furuya, Michale Nitsche, Walter Paulus, Eckart Altenmüller (2014) Surmounting retraining limits in musicians’dystonia by transcranial stimulation. Annals of Neurology 75(5): 700-707
- Marieke van der Steen, Eva Molendijk, Eckart Altenmüller, Shinichi Furuya (2014) Expert pianists do not listen: the expertise-dependent influence of temporal perturbation on the production of sequential movements. Neuroscience 269C: 290-298
- Shinichi Furuya, Ayumi Nakamura, Noriko Nagata (2013) Transfer of piano practice in fast performance of skilled finger movements. BMC Neuroscience 14 (133)
- Andre Lee, Kenta Tominaga, Shinichi Furuya, Fumio Miyazaki, Eckart Altenmüller (2013) Task-specific tremor in violinists: evidence of coactivation in the 3-8 Hz frequency range.Movement Disorders 28(13): 1890-1892
- Shinichi Furuya, Eckart Altenmüller (2013) Finger-specific loss of independent control of finger movements in musician’s dystonia. Neuroscience 247C: 152-163
- Sara Winges, Shinichi Furuya, Nathaniel Faber, Martha Flanders (2013) Patterns of muscle activity for digital coarticulation. Journal of Neurophysiology 110(1): 230-242
- Shinichi Furuya, Eckart Altenmüller (2013) Flexibility of movement organization in piano performance. Frontiers in Human Neuroscience 7:173
- André Lee, Shinichi Furuya, Matthias Karst, Eckart Altenmüller (2013) Alteration in predictability of sensory outcome of motor action in focal hand dystonia. Frontiers in Human Neuroscience 7:172
- Shinichi Furuya, Michael Nitsche, Walter Paulus, Eckart Altenmüller (2013) Early optimization in finger dexterity of skilled pianists: implication of transcranial stimulation. BMC Neuroscience 14:35
- Shinichi Furuya, John Soechting (2012) Speed invariance of independent control of finger movements in pianists. Journal of Neurophysiology 108(7): 2060-2068
- Shinichi Furuya, Tomoko Aoki, Hidehiro Nakahara, Hiroshi Kinoshita (2012) Individual differences in the biomechanical effect of loudness and tempo on upper-limb movements during repetitive piano keystrokes. Human Movement Science 31(1):26-39
- Shinichi Furuya, Martha Flanders, John Soechting (2011) Hand kinematics of piano playing. Journal of Neurophysiology 106(6): 2849-2864
- Shinichi Furuya, Tatsushi Goda, Haruhiro Katayose, Hiroyoshi Miwa, Noriko Nagata (2011) Distinct interjoint coordination during fast alternate keystrokes in pianists with superior skill. Frontiers in Human Neuroscience 5:50
- Shinichi Furuya, Eckart Altenmüller, Haruhiro Katayose, Hiroshi Kinoshita (2010) Control of multi-joint arm movements for the manipulation of touch in keystroke by expert pianists. BMC Neuroscience 11(1):82
- Shinichi Furuya, John Soechting (2010) Role of auditory feedback in the control of successive keystrokes during piano playing. Experimental Brain Research 204(2) 223-237 Shinichi Furuya, Rieko Osu, Hiroshi Kinoshita (2009) Effective utilization of gravity during arm downswing in keystroke by expert pianists. Neuroscience 164(2) 822-831
- Shinichi Furuya, Hiroshi Kinoshita (2008) Expertise-dependent modulation of muscular and non-muscular torques in multi-joint arm movements during piano keystroke. Neuroscience 156(2): 390-402
- Shinichi Furuya, Hiroshi Kinoshita (2008) Organization of the upper limb movement for piano key-depression differs between expert pianists and novice players. Experimental Brain Research 185 (4): 581-593
[病態]
- Quantitative comparison of corticospinal tracts arising from different cortical areas in humans. Usuda N, Sugawara S.K, Fukuyama H,Nakazawa K, Amemiya K, Nishimura Y. Neuroscience Research,2022 Jul 1;S0168-0102(22)00189-4. doi:10.1016/j.neures.2022.06.008. IF: 3.304
- Origin of Multisynaptic Corticospinal Pathway to Forelimb Segments in Macaques and Its Reorganization After Spinal Cord Injury. Ninomiya T, Nakagawa H, Inoue K, Nishimura Y, Oishi T, Yamashita T, Takada M. Front Neural Circuits, 2022 Apr 6;16:847100. doi:10.3389/fncir.2022.847100. eCollection 2022. IF:5.152
- The dorsal premotor cortex encodes the step-by-step planning processes for goal-directed motor behavior in humans. Nakayama Y, Sugawara SK, Fukunaga M, Hamano YH, Sadato N, Nishimura Y. Neuroimage. 2022;256:119221.doi:10.1016/j.neuroimage.2022.119221. Epub 2022 Apr 18. IF:6.556
- A multisynaptic pathway from the ventral midbrain toward spinal motoneurons in monkeys. Suzuki M, Inoue KI, Nakagawa H, Ishida H, Kobayashi K, Isa T, Takada M, Nishimura Y. J Physiol. 2022 Apr;600(7):1731-1752. doi: 10.1113/JP282429. Epub 2022 Feb 17. IF:5.182
- Assessment of safety of self-controlled repetitive trans-vertebral magnetic stimulation. Sasada S, Kadowaki S, Tazoe T, Murayama T, Kato K, Nakao Y, Matsumoto H,Nishimura Y, Ugawa Y. Clin Neurophysiol 2021 Dec;132(12):3166-3176. doi:10.1016/j.clinph.2021.09.016. IF:3.708
- Role of the nucleus accumbens in functional recovery from spinal cord injury. Sawada M, Nishimura Y. Neurosci Res. 2021 Nov;172:1-6. doi: 10.1016/j.neures.2021.04.006. IF:3.304
- Neuroprosthesis to Regain the Volitional Control of Movement. J Rehab Neuroscience. doi:10.24799/jrehabilneurosci.200731
- Changes in beta and high-gamma power in resting-state electrocorticogram induced by repetitive transcranial magnetic stimulation of primary motor cortex in unanesthetized macaque monkeys.Honda Y,Nakamura S, Ogawa K, Yoshino R, Tobler PN, Nishimura Y, Tsutsui KI. Neuroscience Res. 2021 Oct;171:41-48. doi: 10.1016/j.neures.2021.02.002. IF: 3.304
- The Ventral Striatum is a Key Node for Functional Recovery of Finger Dexterity After Spinal Cord Injury in Monkeys. Suzuki M, Onoe K, Sawada M, Takahashi N, Higo N, Murata Y, Tsukada H, Isa T, Onoe H, Nishimura Y. Cereb Cortex. 2020 May 14;30(5):3259- 3270. pii:bhz307. doi: 10.1093/cercor/bhz307. IF:5.357
- Cerebellar outputs contribute to spontaneous and movement-related activity in the motor cortex of monkeys. Sano N, Nakayama Y,Ishida H, Chiken S, Hoshi E, Nambu A, Nishimura Y. Neurosci Res. 2020 Apr 12. pii: S0168-0102(19)30651-0.doi:10.1016/j.neures.2020.03.010. IF:3.304 9.
- Stimulus out put sinduced by subdural electrodes on the cervical spinal cord in monkeys. Kato K, Nishihara Y,Nishimura Y. J Neural Eng. 2020 Feb 5;17(1):016044. doi: 2 10.1088/1741-2552/ab63a3. IF:5.379
- Input-output relations of the spinal locomotor circuitry in humans. Kawai K, Tazoe T, Kanosue K, Nishimura Y. Sport Sci Res, 2019. 16, 49-61.
- Bypassing stroke-damaged neural pathways via a neural interface induces targeted cortical adaptation. Kato K, Sawada M, Nishimura Y. Nat Commun. 2019 10:4699 https://doi.org/10.1038/s41467-019-12647-y. IF:14.919
- Somatosensation Evoked by Cortical Surface Stimulation of the Human Primary Somatosensory Cortex. Kirin S.C., Yanagisawa T, Oshino S, Edakawa K, Tanaka M, Kishima H, Nishimura Y. Front Neuroscience. 2019 Vol13:1019. doi: 10.3389/fnins.2019.01019 IF:5.152
- The somatosensory cortex receives information about motor output. Umeda T, Isa T, Nishimura Y. Science Advances. 2019 Vol.5, no.7, eaaw5388. IF:14.14
- Dynamic Reorganization of Motor Networks During Recovery from Partial Spinal Cord Injury in Monkeys. Chao Z. C., Sawada M, Isa T, Nishimura Y. Cereb Cortex. 2019 Jul 5;29(7):3059-3073. doi: 10.1093/cercor/bhy172. IF:5.357
- Comprehensive analysis of area-specific and time-dependent changes in gene expression in the motor cortex of macaque monkeys during recovery from spinal cord injury. Higo N, Sato A, Yamamoto T, Oishi T, Nishimura Y, Murata Y, Onoe H, Isa T, Kojima T. J Comp Neurol. 2018 May 1;526(7):1110-1130. doi: 10.1002/cne.24396. IF:3.215
- Influence of trans-spinal magnetic stimulation in electrophysiological recordings for closed-loop rehabilitative systems. Insausti-Delgado A, Lopez-Larraz E, Bibian C, Nishimura Y, Birbaumer N, RamosMurguialday A. Annu Int Conf IEEE Eng Med Biol Soc. 2017 Jul;2017:2518-2521. doi: 10.1109/EMBC.2017.8037369. IF:1.041
- Flexible adaptation to an artificial recurrent connection from muscle to peripheral nerve in man. Kato K, Sasada S, Nishimura Y. J Neurophysiol. 2015 Dec 2:115:978-991,2016 doi: 10.1152/jn.00143.2015. IF:2.71
- Function of nucleus accumbens in motor control during recovery after spinal cord injury. Sawada M, Kato K, Kunieda T, Mikuni N, Miyamoto S, Onoe H, Isa T, Nishimura Y. Science, 2 October 2015:Vol. 350 no. 6256 pp. 98-101, DOI: 10.1126/science.aab3825. IF:47.728
- Histological and electrophysiological analysis of the corticospinal pathway to forelimb motoneurons in common marmosets. Kondo T, Yoshihara Y, Yoshino-Saito K, Sekiguchi T, Kosugi A, Miyazaki Y, Nishimura Y, Okano HJ, Nakamura M, Okano H, Isa T, *Ushiba J. Neuroscience. Res. 2015 Jun 17. pii: S0168-0102(15)00136-4. doi:10.1016/j.neures.2015.05.001. IF:3.304
- Temporal Plasticity Involved in Recovery from Manual Dexterity Deficit after Motor Cortex Lesion in Macaque Monkeys. Murata Y, Higo N, Hayashi T, Nishimura Y, Sugiyama Y, Oishi T, Tsukada H, Isa T and Onoe H. J Neuroscience, 7 January 2015, 35(1): 84-95; doi: 10.1523/JNEUROSCI. 1737-14.2015. IF:6.167
- Brain-machine interface to control a prosthetic arm with monkey ECoGs during periodic movements. Morishita S, Sato K, Watanabe H, Nishimura Y, Isa T, Kato R, Nakamura T and Yokoi H. Frontiersin Neuroscience, 12 December 2014 | doi: 10.3389/fnins.2014.00417. IF:5.152
- Plasticity for recovery after partial spinal cord injury – hierarchical organization. Isa T, Nishimura Y. Neuroscience Res. 2014 Jan;78:3-8. doi: 10.1016/j.neures.2013.10.008. IF:3.304
- Proprioceptive information coded by populational sensory afferents. Umeda T, Isa T, Nishimura Y. The Journal of Physical Fitness and Sports Medicine, 2014 3(5), 477-482 IF:1.637
- Phase and magnitude spatiotemporal dynamics of β oscillation in electrocorticography(ECoG) in the monkey motor cortex at the onset of 3D reaching movements. Watanabe H, Takahashi K, Nishimura Y, Isa T. Annu Int Conf IEEE Eng Med Biol Soc. 2014;2014:5196-9. doi: 10.1109/EMBC.2014.6944796. IF:1.041
- Volitional Walking via Upper Limb Muscle-Controlled Stimulation of the Lumbar Locomotor Center in Man. Sasada S, Kato K, Kadowaki S, Groiss SJ, Ugawa Y,Komiyama T, Nishimura Y. J Neuroscience. 2014 Aug 13; 34(33):11131-42. doi: 10.1523/JNEUROSCI.4674- 13. IF:6.167
- Reconstruction of intracortical whisker-evoked local field potential from electrocorticogram using a model trained for spontaneous activity in the rat barrel cortex. Watanabe H, Sakatani T, Suzuki T, Sato MA, Nishimura Y, Nambu A, Kawato M, Isa T. Neurosci Res. 2014 Jul 7; pii: S0168-0102(14)00121-7. IF: 3.304
- Decoding of the spike timing of primary afferents during voluntary arm movements in monkeys. Umeda T, Watanabe H, Sato M, Kawato M, Isa T, Nishimura Y. Front Neuroscience., 2014 May 09; doi: 10.3389/fnins.2014.00097. IF:5.152
- Chen C, Shin D, Watanabe H, Nakanishi Y, Kambara H, Yoshimura N, Nambu A, Isa T, Nishimura Y, Koike Y.. Neuroscience Res. 2014 Apr 13; pii: S0168-0102(14)00047-9. doi: 10.1016/j.neures.2014.03.010. IF: 3.304
- Prediction of hand trajectory from electrocorticography signals in primary motor cortex. Chen C, Shin D, Watanabe H, Nakanishi Y, Kambara H, Yoshimura N, Nambu A, Isa T, Nishimura Y, *Koike Y. PLoS One. 2013 Dec 27; 8(12):e83534. IF:3.24
- Role of Direct vs. Indirect Pathways from the Motor Cortex to Spinal Motoneurons in the Control of Hand Dexterity. Isa T, Kinoshita M, Nishimura Y. Front Neurol. 2013 Nov 19;4:191. doi: 10.3389/fneur.2013.00191. IF:4.086
- Spike-timing dependent plasticity in primate corticospinal connections induced during free behavior. Nishimura Y, Perlmutter SI, Ryan WE, Fetz EE. Neuron. 2013 Dec 4; 80(5):1301-9. doi:10.1016/j.neuron.2013.08.028. IF:18.688
- Differential expression of secreted phosphoprotein 1 in the motor cortex among primate species and during postnatal development and functional recovery. Yamamoto T, Oishi T, *Higo N, Murayama S, Sato A, Takashima I, Sugiyama Y,Nishimura Y, Murata Y, Yoshino-Saito K, Isa T, Kojima T. PLoS One. 2013 May 31;8(5):e65701.doi:10.1371/journal.pone.0065701. IF:3.24
- Effects of early versus late rehabilitative training on manual dexterity after corticospinal tract lesion in macaque monkeys. Sugiyama Y, *Higo N, Yoshino-Saito K, Murata Y, Nishimura Y, Oishi T, Isa T. J Neurophysiology. 2013 Jun; 109(12):2853-65. IF:2.714 31.
- Restoration of upper limb movement via artificial corticospinal and musculospinal connections in a monkey with spinal cord injury. Nishimura Y, Perlmutter SI, *Fetz EE. Front Neural Circuits. 2013 Apr 11; 7:57. doi: 10.3389/fncir.2013.00057. IF:3.342
- Population coding of forelimb joint kinematics by peripheral afferents in monkeys. Umeda T, Seki K, Sato MA, Nishimura Y, Kawato M, Isa T. PLoS One. 2012; 7(10):e47749. doi:10.1371/journal.pone.0047749. IF:3.24
- Prediction of muscle activities from electrocorticograms in primary motor cortex of primates. Shin D, Watanabe H, Kambara H, Nambu A, Isa T, Nishimura Y, Koike Y. PLoS One. 2012;7(10):e47992. doi: 10.1371/journal.pone.0047992. IF:3.24
- Cortical and subcortical compensatory mechanisms after spinal cord injury in monkeys. Nishimura Y, Isa T. Exp Neurol. 2012 May;235(1):152-61. doi:10.1016/j.expneurol.2011.08.013. Epub 2011 Aug 23.IF:5.33
- Genetic dissection of the circuit for hand dexterity in primates. Kinoshita M, Matsui R, Kato S, Hasegawa T, Kasahara H, Isa K, Watakabe A, Yamamori T, Nishimura Y, Alstermark B, Watanabe D, Kobayashi K, Isa T. Nature. 2012 Jul 12;487(7406):235-8. IF:49.962 35.
- Reconstruction of movement-related intracortical activity from micro-electrocorticogram array signals in monkey primary motor cortex. Watanabe H, Sato MA, Suzuki T, Nambu A, Nishimura Y, Kawato M, Isa T. J Neural Eng. 2012 Jun; 9(3):036006. doi:10.1088/1741-2560/9/3/036006. IF:5.379
- Neural substrates for the motivational regulation of motor recovery after spinal-cord injury. Nishimura Y, Onoe H, Onoe K, Morichika Y, Tsukada H, Isa T. PLoS One. 2011;6(9):e24854. doi: 10.1371/journal.pone.0024854. IF:3.24
- Motor command for precision grip in the macaque monkey can be mediated by spinal interneurons. Alstermark B, Pettersson LG, Nishimura Y, Yoshino-Saito K, Tsuboi F, Takahashi M, Isa T. J Neurophysiol. 2011 Jul; 106(1):122-6. IF:2.714
- SPP1 expression in spinal motor neurons of the macaque monkey. Yamamoto T, Higo N, Sato A, Nishimura Y, Oishi T, Murata Y, Yoshino-Saito K, Isa T, Kojima T. Neurosci Res. 2011 Jan; 69(1):81-6. doi: 10.1016/j.neures.2010.09.010. IF: 3.304
- Quantitative inter-segmental and interlaminar comparison of corticospinal projections from the foreoerlimb area of the primary motor cortex of macaque monkeys. Yoshino-Saito K, Nishimura Y, Oishi T, Isa T. Neuroscience 171(4):1164-1179. 2010. IF:3.59
- Neuronal mechanism of mirror movements caused by dysfunction of the motor cortex. Tsuboi F, v, Yoshino-Saito K, Isa T. Eur J Neurosci. 2010 Oct; 32(8):1397-406. doi:10.1111/j.1460-9568.2010.07395.x. IF:3.386
- SPP1 is expressed in corticospinal neurons of the macaque sensorimotor cortex. Higo N, Sato A, Yamamoto T, Nishimura Y, Oishi T, Murata Y, Onoe H, Yoshino-Saito K, Tsuboi F, Takahashi M, Isa T, Kojima T. J Comp Neurol. 2010 Jul 1;518(13):2633-44. doi: 410.1002/cne.22356. IF:3.215 42.
- Increased expression of the growth-associated protein 43 gene in the sensorimotor cortex of the macaque monkey after lesioning the lateral corticospinal tract. Higo N, Nishimura Y, Murata Y, Oishi T, Yoshino-Saito K, Takahashi M, Tsuboi F, Isa T. J Comp Neurol. 2009 Oct 20; 516(6):493-506. doi: 10.1002/cne.22121. IF:3.215
- Asubcortical oscillatory network contributes to recovery of hand dexterity after spinal cord injury. Nishimura Y, Morichika Y,Isa T. Brain. 2009 Mar; 132(Pt 3):709-21. doi:10.1093/brain/awn338. IF:13.501
- Compensatory changes at the cerebral cortical level after spinal cord injury. Nishimura Y, Isa T. Neuroscientist. 2009 Oct;15(5):436-44. doi: 10.1177/1073858408331375. IF:7.52
- Time-dependent central compensatory mechanisms of finger dexterity after spinal cord injury. Nishimura Y, Onoe H, Morichika Y, Perfiliev S, Tsukada H, Isa T. Science. 2007 Nov 16;318(5853):1150-5. IF:47.728
- Differentially expressed genes among motor and prefrontal areas of macaque neocortex. Sato A, Nishimura Y, Oishi T, Higo N, Murata Y, Onoe H, Saito K, Tsuboi F, Takahashi M, Isa T, Kojima T. Biochem Biophys Res Commun. 2007 Oct 26; 362(3):665-9. IF:3.575
[健常]
- Preserving Agency During Electrical Muscle Stimulation Training Speeds up Reaction Time Directly After Removing EMS, Kasahara S, Takada K, Nishida J, Shibata K, Shimojo S, & Lopes P, Proceedings of the 2021 CHI Conference on Human Factors in Computing Systems, 1-9.
- Pain Control by Co-adaptive Learning in a Brain-Machine Interface, Zhang S, Yoshida W, Mano H, Yanagisawa T, Mancini F, Shibata K, Kawato M, & Seymour B, Current Biology, 2020, 30:3935-3944.
- Spatial variability induces generalization in contextual cueing, Higuchi Y, Ueda Y, Shibata K, & Saiki J, Journal of Experimental Psychology: Learning, Memory, and Cognition, 2020, 46(12):2295–2313.
- Neural signals in amygdala predict implicit prejudice toward an ethnic outgroup, Izuma K, Aoki R, Shibata K, & Nakahara K, NeuroImage, 2019, 189:341-352.
- Toward a comprehensive understanding of the neural mechanisms of decoded neurofeedback, Shibata K, Lisi G, Cortese A, Watanabe T, Sasaki Y, & Kawato M, NeuroImage, 2019, 188:539-556.
- Consolidation and reconsolidation share behavioral and neurochemical mechanisms, (*co-first author) Bang JW*, Shibata K*, Frank S*, Walsh EG, Greenlee M, Watanabe T, & Sasaki Y, Nature Human Behavior, 2018, 2:507-513.
- Neural activity in the reward-related brain regions predicts implicit self-esteem: A novel validity test of psychological measures using neuroimaging, Izuma K, Kennedy K, Fitzjohn A, Sedikides C, & Shibata K, Journal of Personality and Social Psychology, 2018, 114(3):343-357.
- Advances in fMRI real-time neurofeedback, (*co-first author) Watanabe T*, Sasaki Y*, Shibata K*, & Kawato M, Trends in Cognitive Sciences, 2017, 21:997-1010.
- Overlearning hyperstabilizes a skill by rapidly making neurochemical processing inhibitory-dominant, Shibata K, Sasaki Y, Bang JW, Walsh EG, Machizawa MG, Tamaki M, Chang LH, & Watanabe T, Nature Neuroscience, 2017, 20:470-475.
- Fear reduction without fear through reinforcement of neural activity that bypasses conscious exposure, Koizumi A, Amano K, Cortese A, Shibata K, Yoshida W, Seymour B, Kawato M, & Lau H, Nature Human Behavior, 2016, 1:0006.
- Neural predictors of evaluative attitudes towards celebrities, Izuma K, Shibata K, Matsumoto K, & Adolphs R, Social Cognitive and Affective Neuroscience, 2017, 12(3):382-390.
- Differential activation patterns in the same brain region led to opposite emotional states, Shibata K, Watanabe T, Kawato M, & Sasaki Y, PLoS Biology, 2016, 14(9): e1002546.
- Learning to associate orientation with color in early visual areas by associative decoded fMRI neurofeedback, Amano K, Shibata K, Kawato M, Sasaki Y, & Watanabe T, Current Biology, 2016, 26(14):1861-1866.
- Neuroimaging evidence for 2 types of plasticity in association with visual perceptual learning, Shibata K, Sasaki Y, Kawato M, & Watanabe T, Cerebral Cortex, 2016, 26(9):3681-3689.
- A small number of abnormal brain connections predicts adult autism spectrum disorder, Yahata N, Morimoto J, Hashimoto R, Lisi G, Shibata K, Kawakubo Y, Kuwabara H, Kuroda M, Yamada T, Megumi F, Imamizu H, Náñez Sr JE, Takahashi H, Okamoto Y, Kasai K, Kato N, Sasaki Y, Watanabe T, & Kawato M, Nature Communications, 2016, 7:11254.
- Age-related declines of stability in visual perceptual learning, Chang LH, Shibata K, Andersen JG, Sasaki Y, & Watanabe T, Current Biology, 2014, 24(24):2926-2929.
- Two-stage model in perceptual learning: toward a unified theory, Shibata K, Sagi D, & Watanabe T, Annals of the NY Academy of Sciences, 2014, 1316:18-28.
- Decoding reveals plasticity in V3A as a result of motion perceptual learning, Shibata K, Chang LH, Kim D, Nanez Sr JE, Kamitani Y, Watanabe T, & Sasaki Y, PLoS One, 2012, 7(8): e44003.
- Resetting capacity limitations revealed by long-lasting elimination of attentional blink through training, Choi H, Chang LH, Shibata K, Sasaki Y, & Watanabe T, Proceedings of the National Academy of Sciences of USA, 2012, 109(30):12242-12247.
- Preference suppression caused by misattribution of task-irrelevant subliminal motion, Shibata K & Watanabe T, Proceedings of the Royal Society B, 2012, 279(1742):3443-8.
- Monocular deprivation boosts long-term visual plasticity, Shibata K, Kawato M, Watanabe T, & Sasaki Y, Current Biology, 2012, 22(9):R291-292.
- Perceptual learning incepted by decoded fMRI neurofeedback without stimulus presentation, Shibata K, Watanabe T, Sasaki Y, & Kawato M, Science, 2011, 334(6061):1413-1415.
- Boosting perceptual learning by fake feedback, Shibata K, Yamagishi N, Ishii S, & Kawato M, Vision Research, 2009, 49(21):2574-2585.
[倫理]
- Nakazawa E. 2007. Neurophilosophy of memory. The Proceeding of The Second BESETO young-scholar Conference of Philosophy: 111–118, Beijing University.
- Nakazawa E. 2009. Personal identity and memory erasure. The Proceedings of the 3rd BESETO Conference of Philosophy: 339–344, UTCP, DALS.
- Nakazawa E. 2010. Memory erasure and the idea of authenticity. The Future of Philosophy in East Asia: The Proceedings of the 4th BESETO Conferfence of Philosophy: 205–212, Seoul National University.
- Fujita M, Hayashi Y, Tashiro S, Takashima K, Nakazawa E, Akabayashi A. 2014. Handling incidental findings in neuroimaging research in Japan: current state of research facilities and attitudes of investigators and the general population. Health Research Policy and Systems 12: 58. doi:10.1186/1478-4505-12-58.
- Nakazawa E, Yamamoto K, Tachibana K, Toda S, Takimoto Y, Akabayashi A. 2016. Ethics of decoded neurofeedback in clinical research, treatment, and moral enhancement. American Journal of Bioethics Neuroscience 7(2): 110–117. doi: 10.1080/21507740.2016.1172134.
- Yasumura A, Takimoto Y, Nakazawa E, Inagaki M. 2016. Decision making in children with attention-deficit/hyperactivity disorder. Open Journal of Pediatrics 6(2): 158–162. doi: 10.4236/ojped.2016.62023.
- Takashima K, Takimoto Y, Nakazawa E, Hayashi Y, Tsuchiya A, Fujita M, Akabayashi A. 2017. Discovery and informing research participants of incidental findings detected in brain magnetic resonance imaging studies: Review and multi-institutional study. Brain and Behavior. 2017 May; 7(5): e00676. https://doi.org/10.1002/brb3.676.
- Fukushi T, Isobe T, Nakazawa E, Takimoto Y, Akabayashi A, Sakura O. 2017. Neuroethics in far east. In Routledge Handbook of Neuroethics, Ed. Johnson LSM, Rommelfanger KS, New York: Taylor & Francis/Routledge, 442–456.
- Toda S, Nakazawa E, Yamamoto K, Akabayashi A. 2018. From “cannot” function to “might” function: Assessment of actual levels of consciousness and potential consciousness in patient care: Japanese experiences. American Journal of Bioethics Neuroscience 9(1): 20–22. doi: 10.1080/21507740.2018.1425762.
- Nakazawa E, Yamamoto K, Akabayashi A. 2018. Fairness and desert: A critique of the random selection criterion in clinical trials. American Journal of Bioethics 18(4): 81–82. doi: 10.1080/15265161.2018.1444818.
- Toda S, Yamamoto K, Nakazawa E, Akabayashi A. 2018. Bridging matters of uncertainty: The importance of focusing on “states in between” for disorders of consciousness. American Journal of Bioethics Neuroscience 9(2): 83–84. doi: 10.1080/21507740.2018.1466836.
- Nakazawa E, Tsuchiya A. 2018. Polarity of public perception over general consent: Survey on consciousness of healthy Japanese participants in brain database projects. Annals of Bioethics and Clinical Applications 1(1): 000102. doi: 10.23880/abca-16000102.
- Rommelfanger KS, Jeong SJ, Ema A, Fukushi T, Kasai K, Ramos KM, Salles A, Singh I, Amadio J, Bi GQ, Boshears PF, Carter A, Devor A, Doya K, Garden H, Illes J, Johnson LSM, Jorgenson L, Jun BO, Lee I, Michie P, Miyakawa T, Nakazawa E, Sakura O, Sarkissian H, Sullivan LS, Uh S, Winickoff D, Wolpe PR, Wu KCC, Yasamura A, Zheng JC. 2018. Neuroethics questions to guide ethical research in the international brain initiatives. Neuron 2018 Oct 10;100(1):19–36. doi: 10.1016/j.neuron.2018.09.021.
- Sadato N, Morita K, Kasai K, Fukushi T, Nakamura K, Nakazawa E, Okano H, Okabe S. 2019. Neuroethical issues of the Brain/MINDS project of Japan. Neuron 101(3): 385–389. doi: 10.1016/j.neuron.2019.01.006.
- Akabayashi A, Nakazawa E, Jecker NS. 2020. Adhering to ethical benchmarks in neurology clinical trials using iPSCs. Neurotherapeutics 17(2): 606-608. doi: 10.1007/s13311-019-00728-1
- Ino Y, Nakazawa E, Akabayashi A. 2019. Health and welfare in Japan. The Lancet 394 (10209):1614–1615. doi: 10.1016/S0140-6736(19)31805-7.
- Akabayashi A, Nakazawa E. Akabayashi A. 2019. Implementation of Japan’s first clinical research regulatory law: Background, overview, and challenges. HEC Forum 31(4): 283–294. doi: 10.1007/s10730-019-09379-3.
- Ino H, Nakazawa E, Akabayashi A. 2020. Drug repurposing for COVID-19: Ethical considerations and roadmaps. Cambridge Quarterly of Healthcare Ethics 30(1): 51–58. doi: 10.1017/S0963180120000481. Epub 2020 Jun 5.
- Nakazawa E, Yamamoto K, London AJ, Akabayashi A. 2021. Solitary death and new lifestyles during and after COVID-19: wearable devices and public health ethics. BMC Medical Ethics 22(1): 89. doi: 10.1186/s12910-021-00657-9.
- Kubota S, Nakazawa E. 2022. Concept and implications of sexual consent for education: A systematic review of empirical studies. Sexual and Relationship Therapy XX(XX):XX–XX. doi: 10.1080/14681994.2022.2039617.
- Nakazawa E, Akabayashi A. 2022. Citizen and patient participation in precision medicine: Epilepsy treatment using brain organoids derived from iPS cells. American Journal of Bioethics Neuroscience 13(2): 138–140. doi:10.1080/21507740.2022.2048737.
- Muraoka K, Takimoto Y, Nakazawa E, Tsuji T, Liu M. 2022. Stroke Survivors’ Experiences and Needs during the Decision-making Process Considering Rehabilitation Options: A Pilot Descriptive Study in Japan. Progress in Rehabilitation Medicine 7: 20220024. Published 2022 May 13. doi:10.2490/prm.20220024.
- Sato H, Fujita M, Tsuchiya A, Hatta T, Mori K, Nakazawa E, Takimoto Y, Akabayashi A. 2022. Disclosing a diagnosis of autism spectrum disorder to pediatric patients without intellectual disability in Japan in early diagnostic stages and associated factors: A cross-sectional study. BioPsychoSocial Medicine XX(XX):XX–XX.

